Engineered Bacteria Could Help Protect “Good” Gut Microbes from Antibiotics
Anne Trafton | MIT
News Office
Antibiotics are life-saving drugs, but they can also harm the beneficial microbes that live in the human gut. Following antibiotic treatment, some patients are at risk of developing inflammation or opportunistic infections such as Clostridiodes difficile. Indiscriminate use of antibiotics on gut microbes can also contribute to the spread of resistance to the drugs.
In an effort to reduce those risks, MIT engineers have developed a new way to help protect the natural flora of the human digestive tract. They took a strain of bacteria that is safe for human consumption and engineered it to safely produce an enzyme that breaks down a class of antibiotics called beta-lactams. These include ampicillin, amoxicillin, and other commonly used drugs.
When this “living biotherapeutic” is given along with antibiotics, it protects the microbiota in the gut but allows the levels of antibiotics circulating in the bloodstream to remain high, the researchers found in a study of mice.
“This work shows that synthetic biology can be harnessed to create a new class of engineered therapeutics for reducing the adverse effects of antibiotics,” says James Collins, the Termeer Professor of Medical Engineering and Science in MIT’s Institute for Medical Engineering and Science (IMES) and Department of Biological Engineering, and the senior author of the new study.
Andres Cubillos-Ruiz PhD ’15, a research scientist at IMES and the Wyss Institute for Biologically Inspired Engineering at Harvard University, is the lead author of the paper, which appears today in Nature Biomedical Engineering. Other authors include MIT graduate students Miguel Alcantar and Pablo Cardenas, Wyss Institute staff scientist Nina Donghia, and Broad Institute research scientist Julian Avila-Pacheco.
Protecting the Gut
Over the past two decades, research has revealed that the microbes in the human gut play important roles in not only metabolism but also immune function and nervous system function.
“Throughout your life, these gut microbes assemble into a highly diverse community that accomplishes important functions in your body,” Cubillos-Ruiz says. “The problem comes when interventions such as medications or particular kinds of diets affect the composition of the microbiota and create an altered state, called dysbiosis. Some microbial groups disappear, and the metabolic activity of others increases. This unbalance can lead to various health issues.”
One major complication that can occur is infection of C. difficile, a microbe that commonly lives in the gut but doesn’t usually cause harm. When antibiotics kill off the strains that compete with C. difficile, however, these bacteria can take over and cause diarrhea and colitis. C. difficile infects about 500,000 people every year in the United States, and causes around 15,000 deaths.
Doctors sometimes prescribe probiotics (mixtures of beneficial bacteria) to people taking antibiotics, but those probiotics are usually also susceptible to antibiotics, and they don’t fully replicate the native microbiota found in the gut.
“Standard probiotics cannot compare to the diversity that the native microbes have,” Cubillos-Ruiz says. “They cannot accomplish the same functions as the native microbes that you have nurtured throughout your life.”
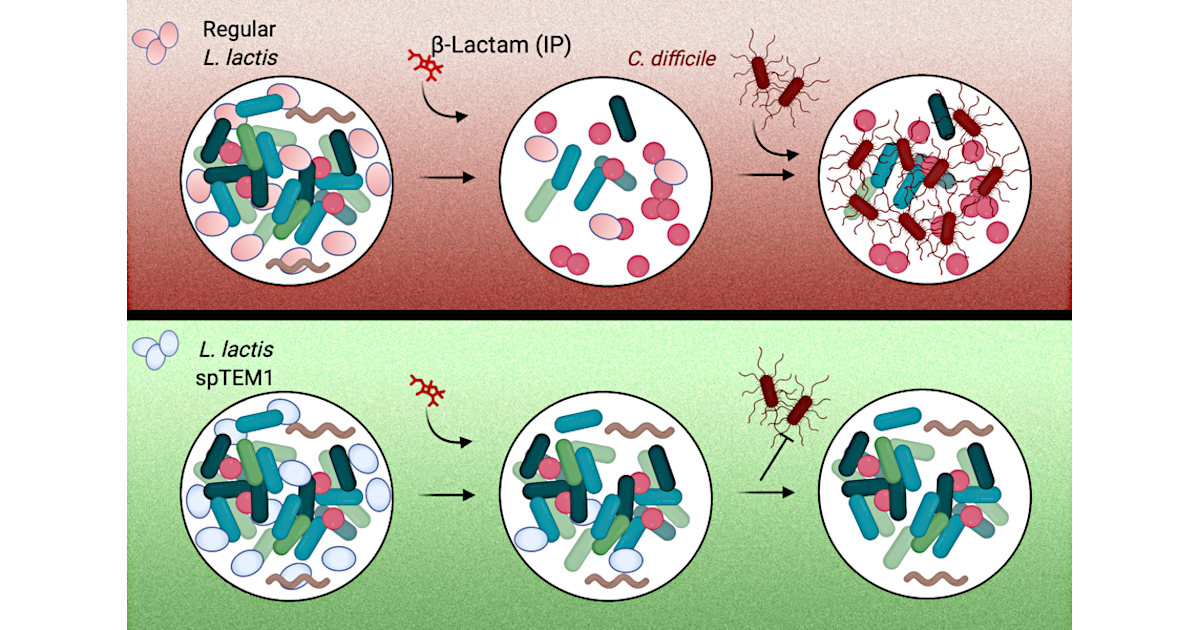
To protect the microbiota from antibiotics, the researchers decided to use modified bacteria. They engineered a strain of bacteria called Lactococcus lactis, which is normally used in cheese production, to deliver an enzyme that breaks down beta-lactam antibiotics. These drugs make up about 60 percent of the antibiotics prescribed in the United States.
When these bacteria are delivered orally, they transiently populate the intestines, where they secrete the enzyme, which is called beta-lactamase. This enzyme then breaks down antibiotics that reach the intestinal tract. When antibiotics are given orally, the drugs enter the bloodstream primarily from the stomach, so the drugs can still circulate in the body at high levels. This approach could also be used along with antibiotics that are injected, which also end up reaching the intestine. After their job is finished, the engineered bacteria are excreted through the digestive tract.
Using engineered bacteria that degrade antibiotics poses unique safety requirements: Beta-lactamase enzymes confer antibiotic resistance to harboring cells and their genes can readily spread between different bacteria. To address this, the researchers used a synthetic biology approach to recode the way the bacterium synthetizes the enzyme. They broke up the gene for beta-lactamase into two pieces, each of which encodes a fragment of the enzyme. These gene segments are located on different pieces of DNA, making it very unlikely that both gene segments would be transferred to another bacterial cell.
These beta-lactamase fragments are exported outside the cell where they reassemble, restoring the enzymatic function. Since the beta-lactamase is now free to diffuse in the surrounding environment, its activity becomes a “public good” for the gut bacterial communities. This prevents the engineered cells from gaining an advantage over the native gut microbes.
“Our biocontainment strategy enables the delivery of antibiotic-degrading enzymes to the gut without the risk of horizontal gene transfer to other bacteria or the acquisition of an added competitive advantage by the live biotherapeutic,” Cubillos-Ruiz says.
Maintaining Microbial Diversity
To test their approach, the researchers gave the mice two oral doses of the engineered bacteria for every injection of ampicillin. The engineered bacteria made their way to the intestine and began releasing beta-lactamase. In those mice, the researchers found that the amount of ampicillin circulating the bloodstream was as high as that in mice who did not receive the engineered bacteria.
In the gut, mice that received engineered bacteria maintained a much higher level of microbial diversity compared to mice that received only antibiotics. In those mice, microbial diversity levels dropped dramatically after they received ampicillin. Furthermore, none of the mice that received the engineered bacteria developed opportunistic C. difficile infections, while all of the mice who received only antibiotics showed high levels of C. difficile in the gut.
“This is a strong demonstration that this approach can protect the gut microbiota, while preserving the efficacy of the antibiotic, as you’re not modifying the levels in the bloodstream,” Cubillos-Ruiz says.
The researchers also found that eliminating the evolutionary pressure of antibiotic treatment made it much less likely for the microbes of the gut to develop antibiotic resistance after treatment. In contrast, they did find many genes for antibiotic resistance in the microbes that survived in mice who received antibiotics but not the engineered bacteria. Those genes can be passed to harmful bacteria, worsening the problem of antibiotic resistance.
The researchers now plan to begin developing a version of the treatment that could be tested in people at high risk of developing acute diseases that stem from antibiotic-induced gut dysbiosis, and they hope that eventually, it could be used to protect anyone who needs to take antibiotics for infections outside the gut.
“If the antibiotic action is not needed in the gut, then you need to protect the microbiota. This is similar to when you get an X-ray, you wear a lead apron to protect the rest of your body from the ionizing radiation,” Cubillos-Ruiz says. “No previous intervention could offer this level of protection. With our new technology we can make antibiotics safer by preserving beneficial gut microbes and by reducing the chances of emergence of new antibiotic resistant variants.”
Suggested Reading
 Opportunities in the Rapidly Growing Pain Management Sector
|
 Stem-Cell Based Therapy for Alzheimer’s Disease
|
 Why Stem Cell Stocks in 2021 Make Sense
|
 Lithium Inflation and Availability Concerns Elon Musk
|
Stay up to date. Follow us:

|